

The Statistical Trending And
Reporting System (STARS) is
a fully validated
software package designed to meet the trending and reporting
needs of the Pharmaceutical
laboratory. STARS is an easy to use 21 CFR Part 11
Compliant application. STARS is a database application that can be
easily configured to work in any commercial database.
STARS also
leverages the power of SAS to perform several key analyses such
as ANOVA, ANCOVA, and Linear Regression.
STARS
contains several modules, which can be
purchased separately, that perform the most common trending and
reporting functions required within Pharmaceutical laboratories. The
four modules are: Stability, Release/SPC, Environmental Monitoring
and Annual Product Review. A description of the base product and
each module is found below.
Common Features to All
Modules
Universal Data Interface
STARS
is
configured with an easy to use LIMS import routine that connects to
all ODBC-compliant LIMS. All that is required is the addition of a
View object within each LIMS for the user identified stability data
fields.
STARS
can
retrieve data from multiple LIMS environments within one project.
STARS is designed to accept stability
data directly from your LIMS but it can also accept stability data
from a CSV-formatted text file, EXCEL file, MS-ACCESS database or SAS dataset.
Data Editing Features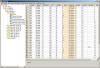
STARS
provides an easy to use Graphical User Interface that allows users
to edit data in a spreadsheet like grid. The most common editing
features are available such as Find/Replace, Cut, Copy, Paste, etc.
STARS
also provides an integrated calculated column feature which allows
users to create Excel-like formula columns.
Statistical/Graphical Analysis
STARS utilizes the powerful SAS statistical analysis software to
easily perform the complex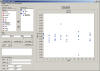 calculation required during stability
data analysis. Here are just a few of the standard calculations
STARS: calculation required during stability
data analysis. Here are just a few of the standard calculations
STARS:
- Analysis of Variance
- Linear Regression
- Weighted Regression
- Scatter Plots
- Residual Plots
- Identification of Potential Outliers
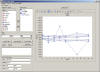
In addition, STARS offers an Adhoc SAS procedure window that
allows users to write and submit their own customized SAS programs.
These Adhoc requests are fully audit trailed.
Adhoc Report Generator
STARS
is configured with an Adhoc Reporting Tool that allows users to
create their own custom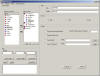 reports. Users can select fields for
reporting, identify sort and group by options as well as a SQL-like
selection language for sub setting datasets. Users can save reports
as templates that can be shared between other users and projects.
Reports can be created in either RTF (Microsoft Word Document), html
or PDF format. STARS
generates many graphical plots to aid in the statistical analysis
process. These plots are created as either PNG or ActiveX files. reports. Users can select fields for
reporting, identify sort and group by options as well as a SQL-like
selection language for sub setting datasets. Users can save reports
as templates that can be shared between other users and projects.
Reports can be created in either RTF (Microsoft Word Document), html
or PDF format. STARS
generates many graphical plots to aid in the statistical analysis
process. These plots are created as either PNG or ActiveX files.
Custom Analysis
STARS
allows users to add their own custom Crystal Report templates or SAS
procedures to a Custom menu. We also provide custom programming
services for more complicated features such as specialized release
and shelf life calculations, custom graphical analyses, and
much more.
21 CFR Part 11 Compliance
STARS has been designed and tested using GAMP guidelines. The
Quality Assurance Program at Integrative Solutions specifies the
production of Validation Master and Project Plans, Configuration Management,
and Change Control. Functional Requirement and Design Specifications have been issued for
STARS. Controlled System
Test Specifications have been executed and Summary
documents have been issued which detail the testing results.
Validation protocols may be audited by appointment at the
Integrative Solutions office in Towaco, NJ.
STARS includes functionality to
meet the Electronic Records and Electronic Signatures rules of 21 CFR Part 11 as they relate to "Closed" systems.
STARS has multiple levels of security
including password expiry. Each user can have different security rights. The full audit trail
includes references to the date and time of the change, the user
that made the change, the type of change made and a description of
the change.
Stability Module Adds These Features to the Base Product:
- Shelf life projections
- Release Limit Calculations
- Trend Analysis
- Outlier Detection
- Homogeneity of Variance Testing
- Projections based on one-sided or two-sided confidence
intervals
- Can vary how limit-of -detection values are handled
- Performs Analysis of Covariance (ANCOVA) for pooling of
multiple studies
- Reports and Graphs can be saved in either RTF, PDF or HTML
formats

Standard and Accelerated Conditions
STARS determines shelf-life for standard conditions using the 95%
confidence interval (upper, lower or two-sided). ANCOVA calculations
for determining the validity of pooling multiple studies which are
assigned a Model I, II, III, or IV designation according to ICH
guidelines.
Trend Analysis
The trend analysis function in
STARS allows you to enter an
expiry period value and calculates a Release Limit range that the
result should be in using either the 95% confidence interval or the
method described in the article "Establishing Shelf Life, Expiry
Limits, and Release Limits" by John Murphy and Jeffrey Hofer, Drug
Information Journal, Vol. 36, pp. 769-781, 2002.
Automatic Model Selection
STARS has been designed to provide an Analysis of Covariance with
an automated model selection routine. All you have to do is enter in
the complete starting model and identify the batch term (if any) and
STARS can determine the final model of significant terms in a few
seconds.
Release/SPC Module Adds These Features to the Base Product:
- Control Charting
- Trend Analysis
- Process Capability Analysis
including all Capability Indices such as Cp, Cpk, Pp, and Ppk
- SPC analysis including X-bar (Mean),
X-bar and Range, X-bar and Standard Deviation, Range, Standard
Deviation, Exception report, Frequency, Distribution, Histogram,
Individuals chart, Moving average chart, Moving average range,
Normality test, NP chart, Out-of-control list, P chart, Pareto
charts, Primary control limits, R and R study, R chart, Robust
chart analysis, CuSum, V-Mask, EWMA
- Reports and Graphs can be saved in either RTF, PDF or HTML
formats
Environmental Monitoring Module Adds These Features to the Base
Product:
-Scheduled Release in 2006
Annual Product Review (APR) Module Adds These Features to the
Base Product:
-Scheduled Release in 2006
Our Customers


Click Here to Request
Additional Information on STARS!
|











